Introduction
Cranial defects may have adverse neurological, aesthetic, and psychological repercussions on patients that may severely hamper the patient’s quality of life.
Aetiology of cranial defects is multifactorial and may occur due to congenital malformations, pathological tumors, trauma, neurosurgical procedures like decompressive craniectomies (DC), tumor resections and infections like osteomyelitis.1
DC often results in the “syndrome of the trephined” (SOT) due to change in cerebral hemodynamics as a result of atmospheric pressure and the resultant effects on the scalp and dura. These may manifest as headaches, confusion, irritability, hemiparesis, epilepsy etc.2
Cranioplasty aims to re-establish the cranial contour, restoring the intracranial physiological pressure and thereby protects the underlying brain. These procedures also improve the aesthetics and minimize patient apprehension.3
For centuries, several materials have been used to cover osseous cranial defects including coconut shells, autografts, allogenic and xenogenic bone grafts, metals and more recently, biosynthetic materials such as resins and ceramics. Polymethylmethacrylate (PMMA) is a very suitable and one of the most extensively used thermoplastic materials that can be prefabricated or even molded intraoperative for prosthetic rehabilitation. 4
This article presents the functional and aesthetic rehabilitation of a patient with Cranioplasty with preoperatively customized Polymethyl methacrylate cranial prosthesis by using 3-Dimensional printed Polylactic acid mold (PLA).
Case Report
A 26-year-old male patient presented to the department of Neurosurgery, INHS Asvini (Naval hospital) for emergency decompressive craniotomy following a severe head injury in a road traffic accident. The patient was kept under observation in the hospital after the surgery and subsequently discharged after two weeks after the DC two weeks following an uneventful recovery.
However, he reported back to the same hospital after three months with continuous headaches and frequent bouts of dizziness. Patient was provisionally diagnosed with “syndrome of the trephined” as a sequel to DC.2 The patient was advised an axial spiral computed tomography (CT) scan with 1-mm thickness and the measurements of the cranial defects were subsequently measures and correlated clinically.
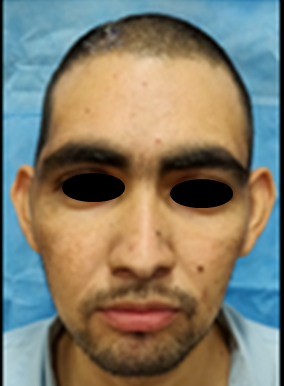
Patient was subsequently referred to the dental department of the hospital for prosthetic rehabilitation of the acquired skull defect. On examination, a skull defect off 12 cm ×8 cm was present in the parietotemporal area of the skull (Figure 1). It was decided to prefabricate a customized PMMA prosthesis using 3D printed mold for rehabilitating the skull defect.
Prosthesis digital design and 3D Printing
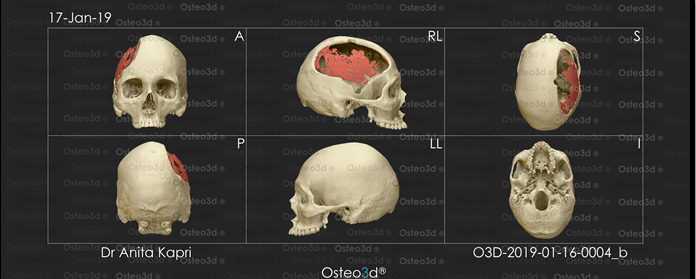
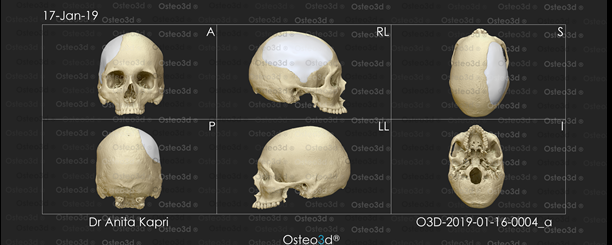
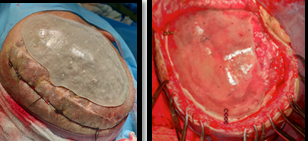
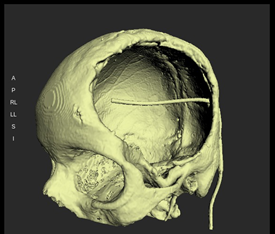
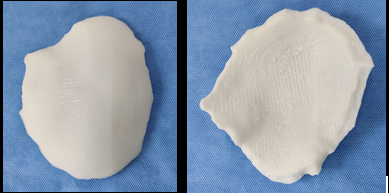
The CT scan data was stored in the standard format DICOM (Digital Images and Communications in Medicine). Through the DICOM viewer, Osteo3D generated a three-dimensional reconstruction of all the CT cross-sectional images. This data was exported as a stereolithography extension file (STL) format using software and a 3D image of the skull was generated (Figure 2). A 3D cranial plate was designed with the help of the software (Figure 3) and the data was transferred to a 3D printer, which fabricated the Polylactic acid (PLA) mold of the designed cranial plate and PLA mold was printed out from the STL file in approximately 30 printing hours. The 3D printed cranial mold was flasked in a larger maxillofacial flask. Mold was then removed from the flask and then the area covered by mold was poured with an antibiotic-coated heat cure PMMA into the flask and following the regular curing cycle of the material, the PMMA cranial plate was removed and a prefabricated PMMA prosthesis was thus acquired. (Figure 4). It was then sterilized in steam autoclave at 134 °C for 5 minutes.
Surgical pacement of cranial implant
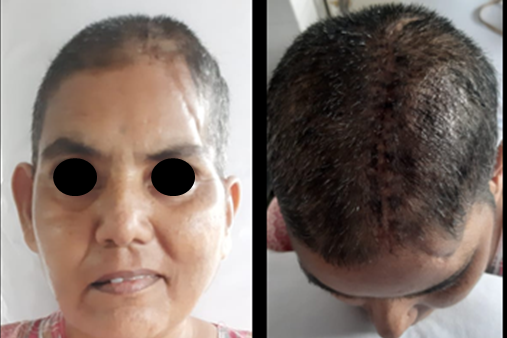
The surgery for insertion of the PMMA prosthesis was performed under general anesthesia and subgaleal skin composite flap was reflected to expose the margins of the calvarial defect. (Figure 5) The prosthesis was stabilized over the calvarial defect using three titanium miniplates. No dural tear was encountered during the surgical procedure; surgical site was seamlessly debrided and closed in layers and a closed circuit surgical drain was inserted. Postoperative care instructions were given, recovery was uneventful and the patient was discharged on the fourth day postoperative (Figure 6). The patients were evaluated every month for six months to register PMMA behavior, biological safety and any eventualities. There were no adverse events such as prosthesis exposure, infections and symptoms of toxicity since the tissues in the region were not exposed to an exothermic reaction of the setting PMMA. After a 6-month follow-up post operatively, the patient had appropriate neurological progression that was defined by measuring his cognitive and motor skills and a satisfactory aesthetic outcome. (Figure 7)
Case 2
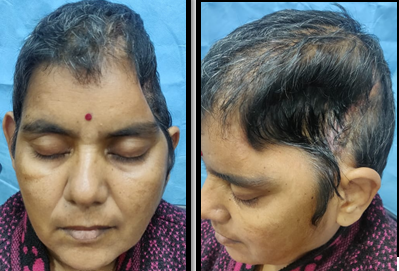
The similar case of mother of a young serving soldier was brought to our institute by her relatives with a chief complaint of fractured skull bone. She was a case of EDH (extradural hematoma) for which he was operated at a civil hospital. Decompressive craniectomy was done which resulted in a residual cranial defect of approx 06x05 inches in the fronto-temporo-parietal region (Figure 8). Due to large size of the defect, the case was planned to be taken up for reconstruction of the defect with PMMA alloplastic implant using 3D printed cranial mould of the defect.
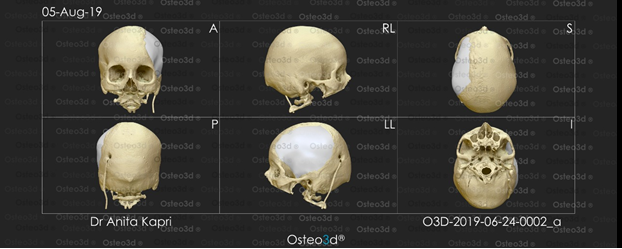
The CT scan data was stored in the standard format DICOM (Digital Images and Communications in Medicine). Through the DICOM viewer, Osteo3D generated a three-dimensional reconstruction of all the CT cross-sectional images. This data was exported as a stereolithography extension file (STL) format using software and a 3D image of the skull was generated (Figure 9).
A 3D cranial plate was designed with the help of the software (Figure 10) and the data was transferred to a 3D printer, which fabricated the Polylactic acid (PLA) mold of the designed cranial plate and PLA mold was printed out from the STL file in approximately 30 printing hours. The 3D printed cranial mold was duplicated in PMMA heat cure similar to the first case with the help of a larger maxillofacial flask (Figure 11). It was then sterilized in steam autoclave at 134 °C for 5 minutes and surgically placed as in the first case. Postoperative care instructions were given, recovery was uneventful and the patient was discharged on the fourth day postoperative (Figure 12).
Discussion
Obtaining good esthetics and an ideal topographical substitute of the calvarial defect during cranioplasty is a daunting task. A wide array of biomaterials including autografts, allogenic and xenogenic bone grafts, metals and more recently, biosynthetic materials such as resins and ceramics is available for prosthetic rehabilitation of a cranial defect.5 Nowadays, both titanium and PMMA are extensively used alloplastic materials as viable alternatives.6 However, titanium is more expensive and difficult to pre-fabricate as compared to PMMA1 and the latter offers the advantages of being inert, radio‐transparent, nonmagnetic, simple to contour, relatively inexpensive and with remarkable plasticity and long-term stability. 7
PMMA implants can be prefabricated or molded intraoperative. However, when in direct contact, intra-operative fabrication of PMMA may adversely affect the Dura and underlying parenchymal tissue when in direct contact by exothermic polymerization reaction and release of toxic residual monomer with compromised aesthetics.8
With the aid of 3D design and printing technology, the size and shape of the defect determined from imaging data can be transferred into planning software to produce a patient-specific implant. The merits of prefabricated PMMA prosthesis are simplicity in procedure, reduced surgical time satisfactory aesthetics and less post-operative infections. Complications of PMMA implant range from, infection, post-operative hematoma, chronic pain, to migration or fracture of cranial prosthesis and may be present in 9-23% of patients.9
SOT or sinking skin flap syndrome “after DC may occur due to neurological deterioration following removal of a large skull bone flap.2 It typically presents as headache pain and dizziness and/or mental depression. Recent studies suggest that cranioplasty contributes to neurological improvement in craniectomized patients.10
Our patient too showed remarkable aesthetic and neurological improvement after the cranioplasty with PMMA prosthesis.
Conclusion
This case report demonstrated the effectiveness of a pre-fabricated, 3D designed customized PMMA cranial prosthesis, which is functionally, economically, aesthetically and biologically acceptable to the patient. The 3D designing and printing techniques can be used successfully to construct patient-tailored cranioplasty implants and negate the potential complications and toxicity of intraoperative PMMA.