- Visibility 40 Views
- Downloads 5 Downloads
- DOI 10.18231/j.aprd.2020.020
-
CrossMark
- Citation
Evaluation of root dentin microhardness prepared with hand and two rotary file systems with 2.5% sodium hypochlorite irrigation solution - an in vitro study
- Author Details:
-
Murali Krishna Raju Saripella
-
Ravi Kumar Konagala *
-
Anupreeta A
-
Lakshman Varma U,
-
Ramesh P
Introduction
“It is not only what is removed, but what is left behind that is important”
Endodontic therapy is essentially a debridement procedure that requires the removal of the irritants from the root canal system and periapical tissue. The need for an irrigating solution during biomechanical preparation is unquestionable. It has been reported that some chemicals used for endodontic irrigation are capable of causing alterations in the chemical composition of dentin. Any change in the calcium: phosphorous ratio may change the original proportion of organic and inorganic components which in turn change the microhardness, permeability and solubility characteristics of dentin.[1] Sodium hypochlorite (NaOCl) is the most commonly used root canal irrigant worldwide. The literature shows that NaOCl treatment removes dentinal organic components and changes their composition.[2], [3]
The introduction of nickel titanium, or NiTi rotary instrumentation by Walia and coworkers(1998) has made endodontics easier and faster than instrumentation with the hand files, resulting in consistent and predictable root canal shaping.[4], [5] Microhardness is sensitive to composition and surface changes of tooth structure. The effect of root canal irrigants on microhardness of root dentin was previously evaluated. [6], [7] Also, it is of interest to investigate the effect of various file types on the microhardness of root dentin. It has been indicated that microhardness determination can provide indirect evidence of mineral loss or gain in the dental hard tissues.[8] Vickers microhardness Tester is used to assess the alterations in root canal microhardness values. The purpose of this study was to determine the effect on root dentin microhardness after instrumentation with hand stainless steel K-files, Protaper and K3 NiTi rotary file systems, along with 2.5% sodium hypochlorite solution as irrigant.
Materials and Methods
Freshly extracted fifty human mandibular single rooted premolar teeth with straight roots which were extracted for periodontal pathologies and orthodontic reasons were included in the study. Selection of teeth was based on their relative dimensions and similarity in morphology. Teeth with caries, root fissures and fracture were excluded. Later the teeth were stored in normal saline in a glass beaker at room temperature till further use. Access opening was done by using Endo access burs (Dentsply Maillefer, Switzerland). The patency of the canals was verified with a no. 10 K-file. To establish the working length of the samples, no.10 K file was inserted into the root canal until it could be just seen through the apical foramen. 1 mm short of anatomic apex was taken as the final working length. After extirpation of the pulp, all the specimens were then randomly divided into five groups with 10 specimens in each group.
GROUP I (Positive control)
In this group, the samples were neither instrumented nor irrigated
GROUP II (Negative control)
In this group, the samples were irrigated with 10 ml of 2.5% sodium hypochlorite (Prime dental products Pvt. Ltd., Thane, Maharashtra, India. Lot No.111130-01) for 1 minute without any instrumentation of the canal.
GROUP III (Stainless Steel K- files)
Root canals were prepared by hand instrumentation with K file (Prime Dental Products, Thane, Mumbai, India) using crown down technique. All canals were prepared with ISO 0.02 taper stainless steel K files. The preparation was done with a no.50 K file working down the canal to a #15 K file. Irrigation with 2.5% Sodium Hypochlorite and recapitulation was done after every instrument. The apical third preparation was done starting with no.20 K file and enlarged upto #25 K file.
GROUP IV (ProTaper Rotary System)
Canals were prepared with ProTaper (Dentsply Maillefer, Switzerland) rotary files in crown down technique. Before the use of protaper files, a glide path with no.20 K file to the set working length was established. Preparation was started with shaper S1 using multiple, passive pressure passes up to three quarters of the estimated canal length. Irrigation with 2.5% NaOCl and recapitulation was done to establish patency to full working length. Preparation was then extended with S1 and S2 shaping files to full working length. Irrigation and recapitulation were done again. Patency of working length was confirmed with #15 hand K-file. This was followed by F1 finishing file to working length. Irrigation and recapitulation was done again. Final preparation was done till F2 (apical size 25) finishing file, between repeated irrigation and recapitulation.
GROUP V (K3 Rotary Instrumentation System)
Canals were prepared with K3 rotary files (SybronEndo, USA) in crown down technique. Before the use of K3 files, a glide path with no.20 K- file to the set working length was established. Initially .08 tapered orifice opener was used 3-4 mm into the canal for coronal pre-flaring. Canal preparation was completed using 0.06 tapered instruments in sequences from the largest to the smallest, starting with size 40, then 35, 30, 25 and 20. This sequence was repeated until size 25 reached working length. Irrigation with 2.5% sodium hypochlorite and recapitulation was done after every instrument. Final irrigation was done with 5ml of distilled water in all the groups except Group I and the canals were dried using paper points. After completion of preparation of samples, the teeth were decoronated. Each root was sectioned transversely into three segments of 3 mm thickness from coronal, middle and apical thirds using diamond disks. For the Microhardness test, the dentin samples were mounted in self curing acrylic resin, held in a aluminium stub ([Figure 1]) 30 samples were obtained from each group and then polished using red mounted fine grained grinding stone followed by silicon carbide and emery abrasive paper of 100 to 800 grit. The microhardness measurements were taken at three different points at a distance of 500 μm and 1000 μm from the lumen of each sample and the average of the 3 readings were taken for both the distances. Each measurement was carried out by using a 300 gram load for 10 seconds, oriented perpendicular to the surface. The microhardness measurements were performed by using a Vickers Microhardness Tester (LEICA VMHT AUTO) in vickers hardness units (VHN), ([Figure 2]).
Results
The mean and standard deviation were calculated for each group. The data was transferred to IBM SPSS software version 20.0 (IBM, Armonk, NY, United States of America) for statistical analysis. The tabulated observations were then statistically analyzed using Analysis of variance technique (One Way ANOVA) to evaluate the difference among five groups followed by Student’s ‘t’ test for pair wise comparison. A “p” value of <.05 was considered for statistical significance. The results shown in [Table 1], demonstrated that mean value of alteration in microhardness (Coronal+Middle+Apical) measured at 500 μm from the lumen, mean difference between the groups, standard deviation and its statistical significance. The Highest mean difference in microhardness values among all the groups was seen between group I and III (12.17 VHN) , and the lowest mean difference was seen between group IV and V (0.13 VHN). ANOVA analysis test demonstrated that biomechanical preparation done with different instrumentation techniques using a common irrigant exhibited statistically significant differences in microhardness values except between Group IV and V. Group I exhibited significantly high microhardness value and Group III showed the least when compared with other groups.
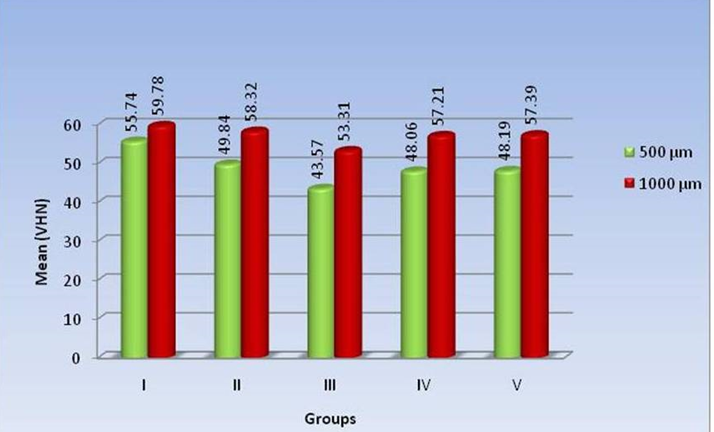
The Mean values of alteration in microhardness measured at a distance of 1000 μm from the lumen, mean difference between the groups, standard deviation and its statistical significance are presented in [Table 2]. Microhardness measurement among different groups showed the highest mean difference between Group I and Group III (6.47 VHN) and lowest mean difference between Group IV and Group V (0.18 VHN) ([Figure 3]).
ANOVA analysis test demonstrated that biomechanical preparation done with different instrumentation techniques using a common irrigant exhibited statistically significant differences in microhardness values except between Group IV and V. Group I exhibited significantly high microhardness value and Group III showed the least when compared with other groups.
The results in [Table 1] [Figure 3] shows comparison of mean values of Microhardness measured at 500 μm & 1000 μm from the lumen, standard deviation & tests of significance. All the five groups showed significantly higher microhardness values at a distance of 1000 μm from the lumen compared to a distance of 500 μm from the lumen. Group III showed highest mean difference with 9.74 VHN and least was in control group with 4.04 VHN.
| Group | Micro hardness (VHN) | Difference between groups | |||||
| Range | Mean | SD | Group Compared | Mean Difference | *t -value | P value | |
| I | 53.16-57.86 | 55.74 | 1.58 | I – II | 5.90 | 13.241 | <0.001 S |
| I – III | 12.17 | 33.048 | <0.001 S | ||||
| II | 46.77 - 52.41 | 49.84 | 1.86 | I – IV | 7.68 | 21.512 | <0.001 S |
| I – V | 7.55 | 20.675 | <0.001 S | ||||
| III | 41.57 - 45.38 | 43.57 | 1.25 | II – III | 6.27 | 15.301 | <0.001 S |
| II – IV | 1.78 | 4.453 | <0.001 S | ||||
| IV | 46.16 -49.72 | 48.06 | 1.15 | II – IV | 1.65 | 4.060 | <0.001 S |
| III – IV | -4.49 | -14.444 | <0.001 S | ||||
| V | 46.08 - 50.14 | 48.19 | 1.23 | III – V | -4.62 | 14.411 | <0.001 S |
| IV – V | -0.13 | -0.413 | 0.681 NS |
| Group | Micro hardness (VHN) | Difference between groups | |||||
| Range | Mean | SD | Group Compared | Mean Difference | *t -value | P value | |
| I | 57.67-62.16 | 59.78 | 1.41 | I – II | 1.46 | 4.612 | <0.001 S |
| I – III | 6.47 | 19.704 | <0.001 S | ||||
| II | 56.14-59.52 | 58.32 | 1.02 | I – IV | 2.57 | 8.263 | <0.001 S |
| I – V | 2.39 | 7.969 | <0.001 S | ||||
| III | 51.7 - 55.17 | 53.31 | 1.12 | II – III | 5.01 | 18.136 | <0.001 S |
| II – IV | 1.11 | 4.633 | <0.001 S | ||||
| IV | 55.85-58.51 | 57.21 | 0.83 | II – IV | 0.93 | 3.842 | <0.001 S |
| III – IV | -3.9 | -15.313 | <0.001 S | ||||
| V | 56.06 -58.86 | 57.39 | 0.85 | III – V | -4.08 | -15.902 | <0.001 S |
| IV – V | -0.18 | -0.838 | 0.406 NS |
| Group | 500 μm | 1000 μm | Significance | ||||
| Mean | SD | Mean | SD | Mean Difference (1000 μm - 500 μm) | *t -value | P value | |
| I | 55.74 | 1.58 | 59.78 | 1.41 | 4.04 | 10.458 | <0.001 S |
| I | 49.84 | 1.86 | 58.32 | 1.02 | 8.48 | -21.906 | <0.001 S |
| III | 43.57 | 1.25 | 53.31 | 1.12 | 9.74 | -31.748 | <0.001 S |
| IV | 48.06 | 1.15 | 57.21 | 0.83 | 9.15 | -35.292 | <0.001 S |
| V | 48.19 | 1.23 | 57.39 | 0.85 | 9.20 | -33.779 | <0.001 S |


Discussion
Success in Endodontic therapy depends on chemomechanical debridement of the root canal system using effective instruments and irrigating solutions. Sodium Hypochlorite (NaOCl) has been widely recommended as an irrigant for chemomechanical debridement of root canals due to its solvent activity for necrotic and vital tissues, besides its ability as an effective agent against a broad spectrum of bacteria.[9]
Sayani et al showed that both 5% and 2.5% NaOCl have similar capacity in dissolving the organic tissue and the use of 2.5% NaOCl might be less detrimental to root dentin in terms of surface decalcification. Hence 2.5% NaOCI was used in this study. The degree of mineralization and amount of hydroxyapatite in the intertubular substance are considerable factors in determining the intrinsic hardness profile of dentin structure. Microhardness determination can provide indirect evidence of mineral loss or gain in dental hard tissues.[8] Vickers microhardness test was used in this study because previous studies have shown the suitability and practicability of the vickers microhardness test for evaluation of surface changes of dental tissues treated with chemical agents.[3] The present study was carried out to evaluate the effect on microhardness of root dentin prepared with three different file systems using 2.5% NaOCl as common irrigating solution. Results showed that there was a statistically significant (P<.05) difference between the inner (500 μm) and the outer ring (1000 μm) among all the groups. There was no significant difference between the two rotary systems tested in altering the microhardness values. Group I showed the highest microhardness both at 500 μm and at 1000 μm followed by group II,V,IV and III.
Group II (2.5% NaOCl) showed significantly less microhardness values of root dentin than Group I and significantly high microhardness values when compared to Group III, Group IV and Group V. Slutzky Goldberg et al (2002), studied the microhardness of root dentin after instrumentation with different file types by using 2.5% NaOCl as the irrigating solution and results had shown that 2.5% NaOCl alone had altered microhardness of dentin significantly. [10]
Group III showed significantly less microhardness values than Group IV and V. The working time required with stainless steel files was longer than Nickel-Titanium rotary files.[11], [12] This could be due to the longer exposure of dentin mainly inner dentin to NaOCl due to which its effect on dentin microhardness was more pronounced. Its effect on dentin microhardness was more pronounced.
Group IV and V showed no significant differences between them. This could be due to faster preparation of the root canals. Therefore, the exposure of dentin, mainly the inner dentin to NaOCl was less and its effect on dentin microhardness was less pronounced. [10], [13] Volume of total dentin removal and amount of total dentin removal at all separate horizontal regions were comparable between ProTaper and K3 according to Bergmans et al. (2003) and Akhlaghi et al (2008).[14], [15]
All groups showed significantly higher microhardness values at a distance of 1000 μm from the lumen compared to a distance of 500 μm from the lumen. This is in accordance with the study done by Slutzky-Goldberg et al (2002), Saleh et al (1999) which indicated that the efficacy of the irrigation solution depends on its ability to penetrate and wet intertubular dentin. As the distance the fluid has to penetrate increases, its effects will be reduced. This happens also because the number and size of the dentinal tubules decrease in the periphery. [6], [10]
Pashley et al. reported an inverse correlation between dentin microhardness and tubular density. The tubular density of root dentine close to the lumen is higher than at its periphery. When tubular density increases, dentin micro hardness decreases. This is due to a decrease in the amount of inter tubular dentin and an increase in the individual tubular diameter towards the pulp thus, the increased number of opened dentinal tubules free of peritubular dentin near the pulp, offer little resistance to the testing indenter. All of these factors probably contribute to the decreasing microhardness as the pulp is approached. [16]
According to mannocci et al the number of dentinal tubules in root dentin has been evaluated in extracted teeth. It has been reported to vary from 49,000 to 57,000 per mm2, progressively decreasing in from the crown towards the apical region. Moreover apical dentin is more calcified than cervical or middle root dentin.[17] According to Pecora et al NaOCl was not effective in the apical region as it was in the coronal & middle third probably because it has shown to be less effective in reducing the surface tension at the apical region.[18] All these factors justify the results of the present study having the microhardness of apical root dentin to be higher than coronal root dentin.
Conclusion
Within the limitations of this study, it can be concluded that
The Microhardness of root dentin was significantlycompromised with instrumentation (Manual and Rotary)and irrigation with 2.5% Sodium Hypochlorite.
Manual preparation with stainless Steel K-filesshowed the maximum reduction in microhardness of root dentin.
Preparation of teeth with NiTi rotarysystems(ProTaper & K3) reduced the microhardnessto a lesser extent when compared with Stainlesssteel K-files, using 2.5% Sodium Hypochlorite as acommon irrigant.
The effect of instrumentation and irrigation on rootDentin Microhardness decreased as we move awayfrom the lumen(Pulpo-Dentin interface).
The Microhardness of apical third of root was notmuch affected either with instrumentation or irrigationwhen compared with coronal & middle thirds.
Source of Funding
None.
Conflict of Interest
None.
References
- H Dogan, S Calt. Effects of Chelating Agents and Sodium Hypochlorite on Mineral Content of Root Dentin. J Endod 2001. [Google Scholar]
- S. V. Barbosa, K. E. Safavi, L. S. W. Spangberg. Influence of sodium hypochlorite on the permeability and structure of cervical human dentine. Int Endod J 1994. [Google Scholar]
- I Slutzky-Goldberg, M Maree, R Liberman. Effect of Sodium Hypochlorite on Dentin Microhardness. J Endod 2004. [Google Scholar]
- AWK Chan, Gsp Cheung. A comparison of stainless steel and nickel-titanium K- files in curved root canals. Int Endod J 1996. [Google Scholar]
- G. B. Yang, X. D. Zhou, H. Zhang, H. K. Wu. Shaping ability of progressive versus constant taper instruments in simulated root canals. Int Endod J 2006. [Google Scholar]
- A.A Saleh, W.M Ettman. Effect of endodontic irrigation solutions on microhardness of root canal dentine. J Dent 1999. [Google Scholar]
- Luciane Dias Oliveira, Cláudio Antonio Talge Carvalho, Willian Nunes, Marcia Carneiro Valera, Carlos Henrique Ribeiro Camargo, Antonio Olavo Cardoso Jorge. Effects of chlorhexidine and sodium hypochlorite on the microhardness of root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007. [Google Scholar]
- J. Arends, J.J. ten Bosch. Demineralization and Remineralization Evaluation Techniques. J Dent Res 1992. [Google Scholar]
- Michael J. Jeansonne, Robert R. White. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod 1994. [Google Scholar]
- I Slutzky-Goldberg, R Liberman, I Heling. The Effect of Instrumentation with Two Different File Types, Each with 2.5% NaOCl Irrigation on the Microhardness of Root Dentin. J Endod 2002. [Google Scholar]
- Peter T. Esposito, Charles J. Cunningham. A comparison of canal preparation with nickel-titanium and stainless steel instruments. Journal of Endodontics 1995. [Google Scholar] [Crossref]
- Jeffrey A. Short, Leslie A. Morgan, J. Craig Baumgartner. A comparison of canal centering ability of four instrumentation techniques. J Endod 1997. [Google Scholar]
- C Dayal, G S Sajjan, T S Subash. An in-vitro evaluation of microhardness of root dentin prepared with different file types with 2.5% sodium hypochlorite. Endodontology 2007. [Google Scholar]
- L. Bergmans, J. Van Cleynenbreugel, M. Beullens, M. Wevers, B. Van Meerbeek, P. Lambrechts. Progressive versus constant tapered shaft design using NiTi rotary instruments. Int Endod J 2003. [Google Scholar]
- A Mohammadzade, K Zohreh, L D Mohajeri, M S Sheikholeslami. Comparison of canal preparation pattern of K3 and ProTaper rotary files in curved resin blocks. Iran Endod J 2008. [Google Scholar]
- David Pashley, Atsuko Okabe, Phillip Parham. The relationship between dentin microhardness and tubule density. Endod Dent Traumatol 1985. [Google Scholar]
- T. P. C. Sim, J. C. Knowles, Y-L. Ng, J. Shelton, K. Gulabivala. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int Endod J 2001. [Google Scholar]
- J D Pecora, M D Sousa Neto, Dmz Guerisoli, M A Marchesan. Effect of reduction of the surface tension of different concentrations of sodium hypochlorite solutions on radicular dentine permeability. Braz Dent J 1998. [Google Scholar]
